Details of the Drug
General Information of Drug (ID: DMH7GN8)
| Drug Name |
Ramosetron
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
132036-88-5; UNII-7ZRO0SC54Y; Ramosetron [INN]; 7ZRO0SC54Y; CHEMBL1643895; Ramosetron (INN); (1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone; Nor-YM 060; SCHEMBL16701; GTPL2301; DTXSID0043842; NTHPAPBPFQJABD-LLVKDONJSA-N; MolPort-019-991-383; CHEBI:135156; ZINC5116719; AC1L3355; BDBM50334454; 8235AH; AKOS015896003; SB19072; DB09290; SC-92398; AJ-53160; LS-187182; TL8000762; R-146; FT-0651831; D08466; A806353; (-)-(R)-1-Methylindol-3-yl-4,5,6,7-tetrahydro-5-benzimidazolyl ketone; Nasea (TN); YM060
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
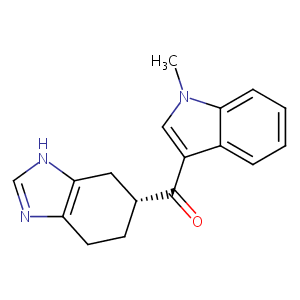 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 279.34 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
